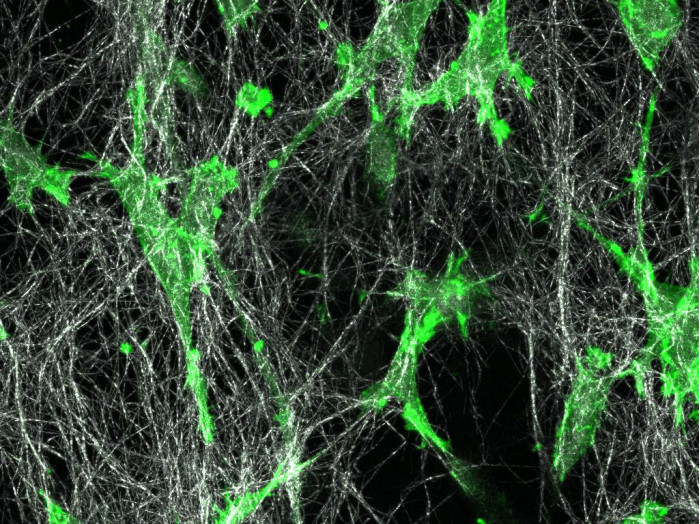
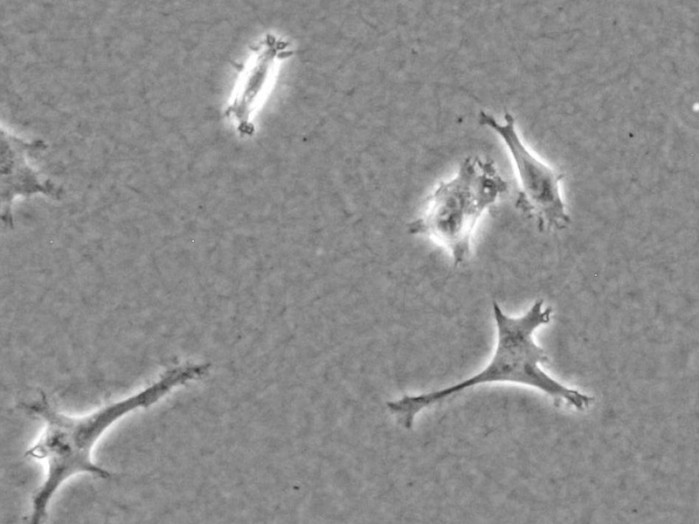
A high-quality collagen type I (bovine or rat tail origin) for cell culture applications (e.g., 3D gels, scaffolds, and coating)
Applications
- 3D cell culture in in vivo-like matrices
- Live cell imaging in a 3D environment
- Creating a 3D scaffold for organoids and spheroids
- Preparation of 3D collagen gels following Application Note 26: "Preparation of Collagen I Gels" (PDF)
- Chemotaxis assays in 3D collagen gels using the µ-Slide Chemotaxis
- Cell migration assays of suspension cells (e.g., leukocytes)
- Coating of cell culture vessels with a thin collagen layer to promote cell attachment
- Cell differentiation studies in a 2D versus 3D environment
- Highly suited for fluorescence microscopy due to low autofluorescence
Technical Features
- High-quality purified protein from rat tendons or bovine hides
- Highly standardized manufacturing process with stringent quality control
- Mild manufacturing process for highest levels of nativity
- Detailed protocol for easy handling in Application Note 26: "Preparation of Collagen I Gels" (PDF)
- Shipping on cool packs for well-defined quality and reproducibility
- Available in 2 concentrations: 5 mg/ml for standard applications and a high-concentrated solution with 10 mg/ml for special applications
- Highest level of natural cross-linking on the market (highest quality grade)
- High viscosity: special pipettes for highly viscous solutions are recommended
- Low autofluorescence
Using Collagen as an in Vivo-Like Matrix for 3D Cell Culture
Collagen is the main component of connective tissue and is abundant in the mammalian body. It is widely used in 3D cell culture for modeling biological extracellular matrix, or as a coating for promoting cell attachment.
Many cells naturally grow in a three-dimensional environment. When cultured in vitro, cells are attached to a flat 2D surface and might behave differently than when they are inside a 3D gel matrix. In many cases, a 3D environment more closely resembles an in vivo situation, which should be considered when planning cell culture assays.
The Collagen Structure
Collagen is a fibrous protein that consists of three α-chains. They combine to create a rope-like triple helix, thus providing tensile strength to the extracellular matrix (ECM). The α-chains contain GXY repeats that include glycine (G), which is an amino acid that fits well into the triple helix. Typically, X and Y represent hydroxyproline and proline, which are key components for collagen stability. The triple helices aggregate and form fibrils in a self-organized manner. In vivo, the fibrils gelate into fibers to form tissue such as tendon or dermis.
The collagen that is used for in vitro experiments is acid- or enzyme-extracted from animal tissue. Under acid conditions (low pH), the fibers dissociate into soluble fibrils. In some extraction methods, the enzyme pepsin is used to facilitate the collagen extraction. However, pepsin also digests the collagen to a certain extent, which leads to a significant decrease in native fiber crosslinking activity.
Unlike pepsin-extracted collagen (usually from bovine skin), the ibidi Collagens are acid-extracted. This, and the very mild manufacturing process, preserve a maximal nativity.
Collagen Gelation
In an acidic solution, the collagen fibrils exist in an unoriented way. Only hydrogen bonds form between the collagen fibrils, which give the solution its high viscosity. Changing to a neutral pH causes the fibrils to gelate into fibers. This creates a three-dimensional collagen gel. The level of nativity of the collagen used defines the level of fiber cross-linking in the collagen network. Native (non-pepsin extracted) collagens exhibit a high level of crosslinking, while pepsinized collagen solutions form a less in vivo-like gel.
The ibidi collagens have a very high viscosity due to the high protein concentration, especially at 10 mg/ml. The pH-driven gelation changes its characteristic properties as follows:
| Non-Gelated Collagen | Gelated Collagen | |
| Macroscopic optical appearance | Clear to slightly opalescent | White, turbid |
| Microscopic optical appearance | Clear, no structures | Network of fibrils, building fibers that are visible under the microscope |
| Viscosity | Very high, but still flowing | Not flowing; small deformations (e.g., by pipet tips) will rupture the structure |
| Pipetting | Can be pipetted with special pipettes for highly viscous liquids | Defined pipetting is not possible, any mechanical force will destroy the structure and the mechanical properties |
What Are the Differences Between ibidi’s Collagen Type I, Rat Tail
and Collagen Type I, Bovine?
Generally, the Collagen Type I, Rat Tail and the Collagen Type I, Bovine from ibidi are equally suitable for experiments requiring a collagen-based matrix and can be chosen based on personal preference. Since both types are acid-extracted and non-pepsinized, the main difference is the species and the tissue, from which the collagens are extracted. There are small differences in the optical gel appearances. Typically, a rat tail gel exhibits a denser fiber network with less prominent fibers and a smaller pore size. A bovine gel is usually easier to visualize in the phase contrast microscope because the fibers are more bundled, which leads to a larger pore size. Further differences are found in the table of specifications above.
Click here to access the ;ibidi Collagen Calculator, a free software tool, designed to simplify the process of creating collagen gel protocols.